EXPERIENCE & REFERENCES
More than 10 years experience in the field of clinical studies:
- Quality management
- Study development
- Study preparation
- Projectmanagement
- Monitoring / SDV
- Study termination
- Study evaluation and reporting
- Patient recruitment
Independent management of international multicenter studies:
in Austria and Germany concerning the following medical indications:
- Oncology: CML, breast cancer, prostate cancer, lung cancer, renal cancer, ovarian cancer, SCCHN, colorectal CA,
myelofibrosis, AML,CLL,DLBCL, hepatocellular carcinoma, multiple myeloma, melanoma - Rheumatic diseases: arthrities, gonarthrosis, coxarthrosis, juvenile idiopathic athritis
- Cardiovascular diseases: angina pectoris, hypertension, peripheral vascular diseases, Pulmonary Hypertension, CTEPH
- Metabolic diseases: diabetes mellitus
- Neurology: cerebrovascular diseases, multiple sclerosis
- Infective diseases: pneunomia, bronchitis
- Immunology: renal transplant, liver transplant, cystic kidney
- Gynaecology: post menopause
Management of investigator initiated trials (IITs) and academic studies:
Oncology: Colorectal CA, melanoma, ovarian carcinoma, peritoneal carcinoma, basal cell carcinoma
Management of non-interventional studies (NIS):
Metabolic diseases: diabetes mellitus, Crohn's disease
Cardiovascular diseases: acute coronary syndrome, heart failure
Neurology: Sexual dysfunction in depression, multiple sclerosis
Oncology: patient registry for NSCLC, MammaCA, renal cell CA, CML, melanoma
Management of Medical Device Studies:
- Cartilage Transplantation
- Hip Joint Replacement
- Wound Healing Support Systems
- Contraception / Copper Coil
- Wound Dressings
- Nasal Sprays
Customers:
Our customers include:
- International pharma companies
- Austrian corporate headquarters
- Corporate headquarters abroad
- National pharma companies
- International CROs
- Academic research groups
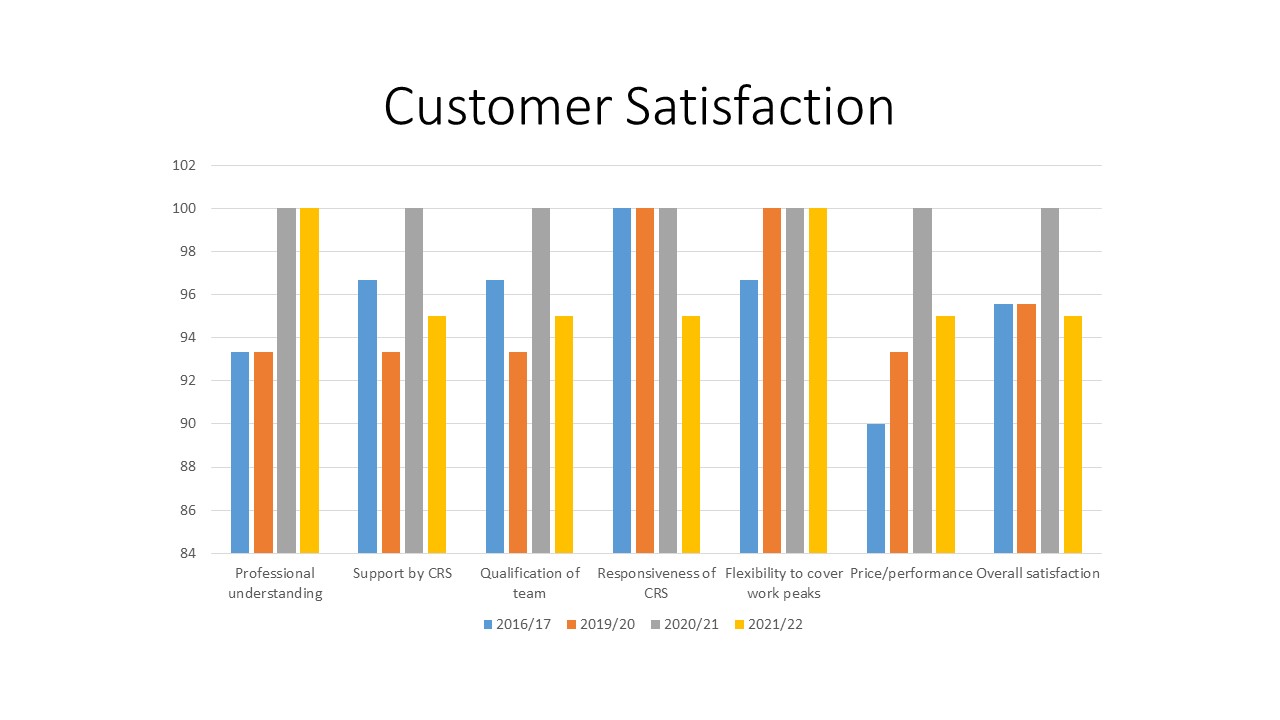
Customer satisfaction
After termination of each study a customer survey is carried out.